Roohealthcare.com – There is not a lot of data available on the Lima SMR Stemless implant. While it is widely used in Europe, FDA approval for the US is pending. There is also no published clinical trial results. Nonetheless, this implant may be a viable option for people with limited bone density. Here’s a quick overview of its pros and cons. And, of course, you can always call your surgeon for a second opinion.
Primary Total Shoulder Joint Replacement
This study is designed to identify patients with total, primary shoulder joint replacement. It is intended to help reduce pain and restore shoulder articular mobility function. Researchers have conducted a prospective multicenter randomized controlled trial to evaluate the device’s efficacy and safety. The goal of the study is to demonstrate non-inferiority to the SMR Reverse Shoulder System for joint replacement. If you’re interested in learning more about this revolutionary device, keep reading!
The Lima SMR Stemless Reverse Shoulder System is an innovative bone-sparing endoprosthesis. It is currently available in Europe, Mexico, and select APAC markets. It has been designed to address severe rotator cuff defects and restore shoulder function. The SMR Stemless Reverse Shoulder System has been cleared by the FDA and LimaCorporate plans to enroll 200 patients in a seven-site study in the USA. The trial will last for up to two years.
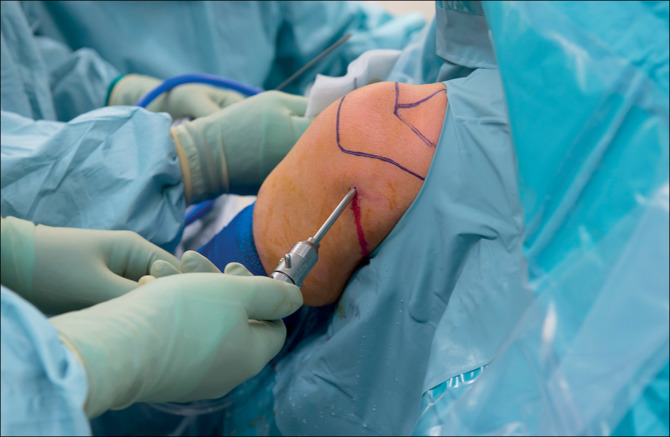
The Lima SMR Stemless has a proven track record. There have been no reported implant-related complications associated with this prosthesis. It has also been evaluated radiologically and functionally. Its minimal bone loss, however, raises concerns about the safety of this procedure. While the Lima SMR is a highly effective treatment option, it may not be right for everyone. It is best suited for people with moderately deficient bone density.
Stemless Humeral Implant
The Lima Shoulder Modular Replacement system was introduced in 2015. The system includes four parts for anatomic and two parts for reverse configuration. The humeral core component is made of trabecular titanium and features 2 fins radiating from the central peg. The humeral core is seated in the metaphysis through impaction. The metallic reverse liner is coupled to the humeral core. This component is an anatomically-corrected, stemless humeral implant.
In the study, 47 patients had anatomic total shoulder arthroplasty for osteoarthritis with the SMR Stemless shoulder system. At a minimum follow-up of 24 months, the reviews were conducted. There were 18 male patients and 22 female patients. The average patient age was 67 years. Standardized radiographs were reviewed for loosening of the joint or radiolucency of the implant. Radiographs were also evaluated for loss of bone density and superior migration of the humeral head.
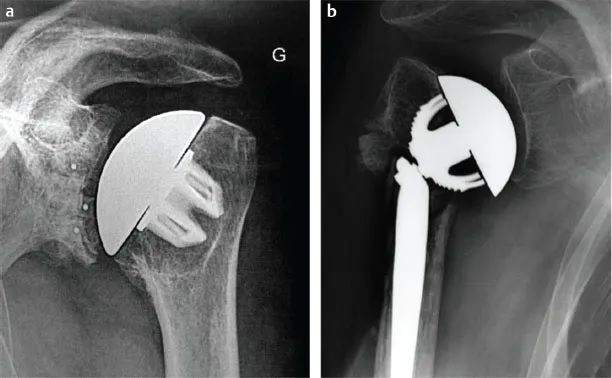
The was a successful revision for failed TESS anatomic prosthesis. The clinical results were comparable to other stemless designs. There was no evidence of device-related complications. Another advantage of this implant is that it’s convertible, making future revisions easier. A surgeon can even change the prosthesis at any time. It’s a good choice for patients with limited bone availability. However, patients should consider their specific situation before making the decision.
Modifiable Stemless Design Implant
The Lima SMR Stemless Reverse Implant was evaluated for its safety and effectiveness in both short and long-term follow-ups. The study period covered the period from January 2016 to November 2018. It included 59 patients undergoing stemless total shoulder arthroplasties. Of these 56, 52 had a stemless implant. During this period, 52 of the patients were followed for a minimum of 24 months.
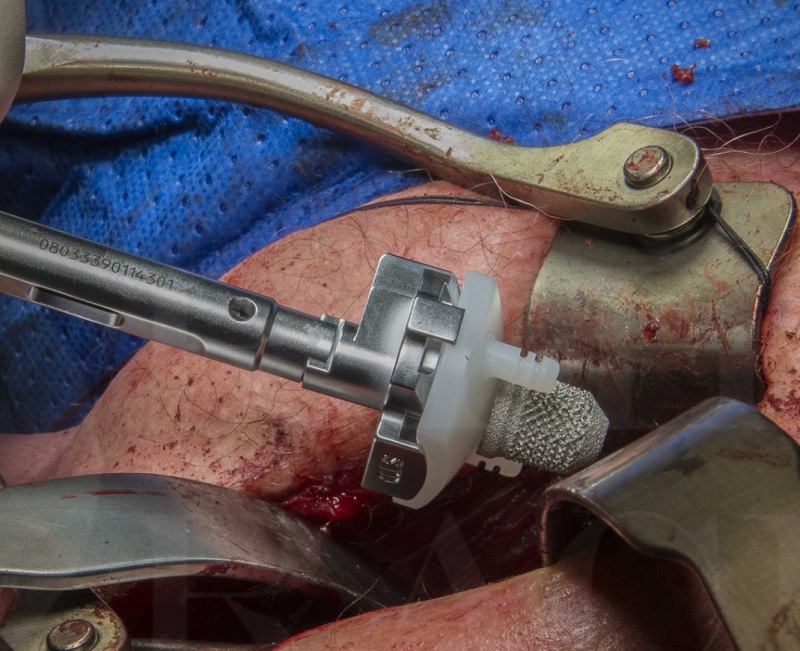
The SMR Stemless implant was approved by the FDA in March 2015. It was initially marketed in Europe in 2004. It was later developed and marketed as the Eclipse. This implant is a convertible stemless design, which lets the surgeon use it in either an anatomic or reverse configuration. The TESS system consists of three components. The first component is a six-armed porous-coated implant implanted into the metaphyseal bone. The second part of the implant is inserted through the female Morse taper.
Reference: